The Process of Biospecimen Procurement – A Modern Step-by-Step Guide
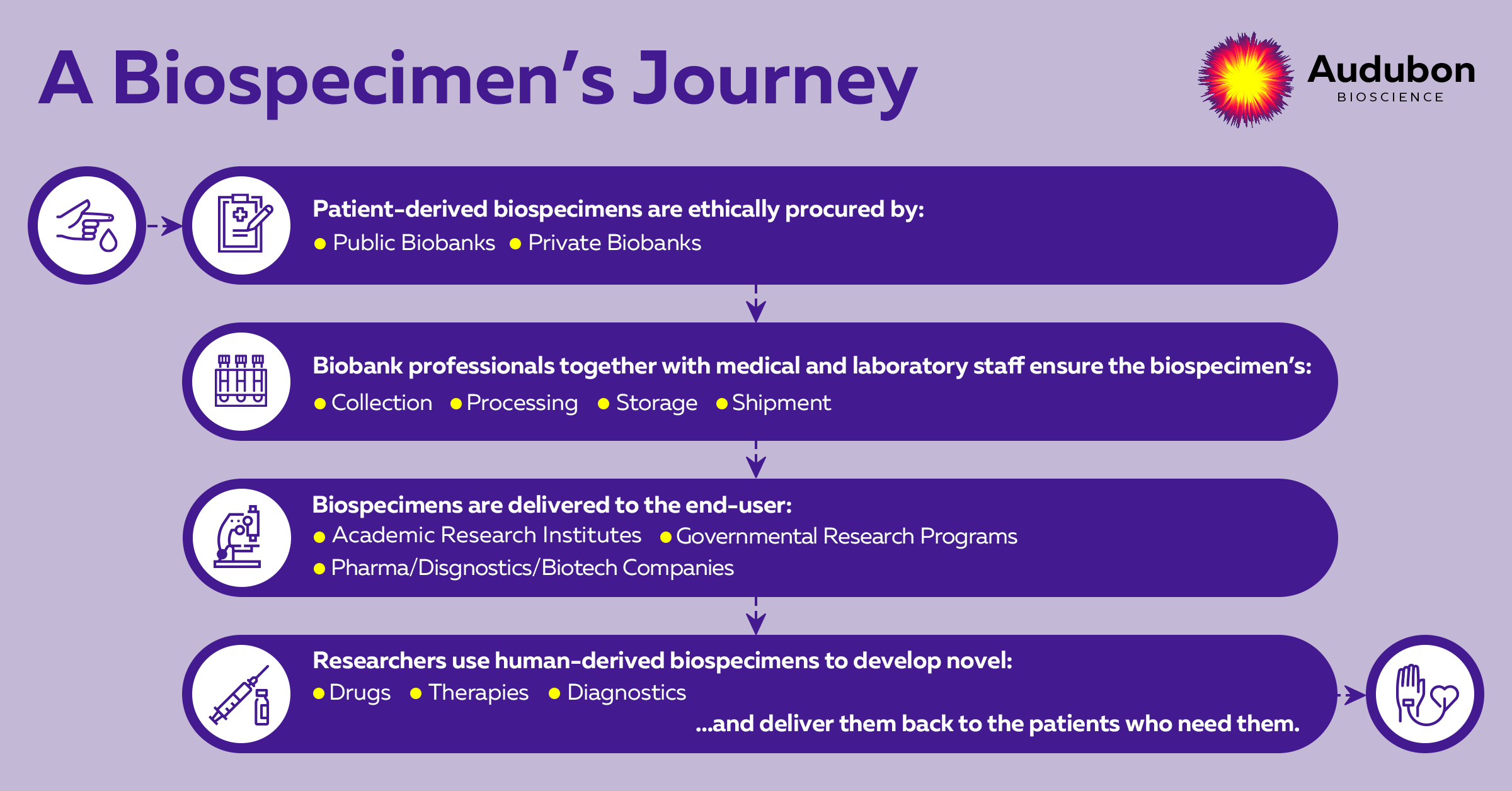
Biospecimens have been essential in biomarker discovery as far back as the 1980s. Biospecimen procurement is the systematic process of acquiring, storing, and managing biosamples for research for the advancement of personalized medicine and drug development, as shown in Figure 1. However, despite its importance, the process of biobanking, particularly prospective biospecimen collection, has been fraught with several challenges, including difficulties with sample quality, collection, and storage.
This article provides a comprehensive guide on modern sample collection that will serve as a practical reference for researchers and professionals hoping to successfully navigate the biospecimen collection process.
Step 1: Planning and Protocol Development
Define Research Objectives
A study’s objectives must first be clearly defined prior to research biospecimen acquisition to ensure collection procedures are in alignment with the broader goals of the study in question. The research purpose must also specify the type of study the procured samples will be utilized for.
Ethical Approval
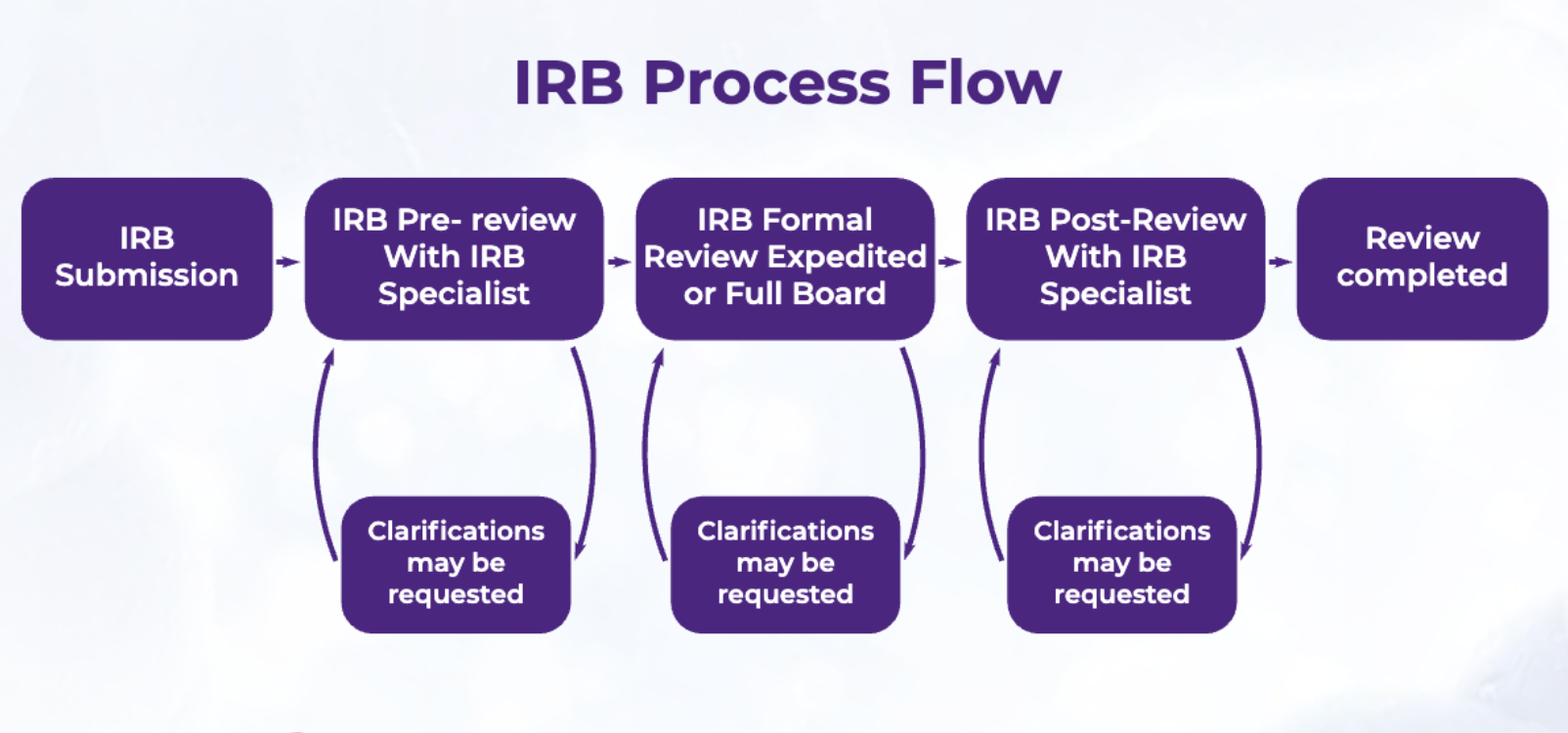
Biospecimen collection procedures are required to comply with all applicable regulatory guidelines enforced by bodies, such as Institutional Review Boards (IRBs), to protect donor rights, welfare, and privacy. All key study-related documents, including the informed consent form, must first receive approval from the IRB, following the review process shown in Figure 2. This Nature editorial covers the story of Henrietta Lacks and her cancer tissue samples, underscoring the importance of the ethical collection of biosamples for research.
At Audubon Bioscience, we follow a strong bioethics policy with the highest ethical standards in conducting prospective biospecimen collection; visit our website to learn how we are supporting our clients’ research with ethically-obtained biospecimens.
Step 2: Participant Recruitment and Informed Consent
Identify Target Participants
Once IRB approval has been received, study-related activities are permitted to begin. There are two key factors to consider while identifying prospective participants:
- Defining Eligibility Criteria: The eligibility criteria should be clearly chosen to ensure the biospecimens collected are aligned with the predefined research objectives.
- Considering Diverse Demographics: To gather robust and generalizable data, participants should be recruited from diverse demographic backgrounds.
Informed Consent Process
The informed consent process involves explaining the purpose of the study, the procedures that will be conducted, potential risks and benefits, and participant rights. Particularly with prospective collection, wherein excess specimens are taken for research purposes, donors are required to provide informed consent in writing prior to procurement. This recent report from the National Institute of Health provides additional guidance on obtaining informed consent for research involving biospecimens.
Step 3: Biospecimen Collection
Collection Methods
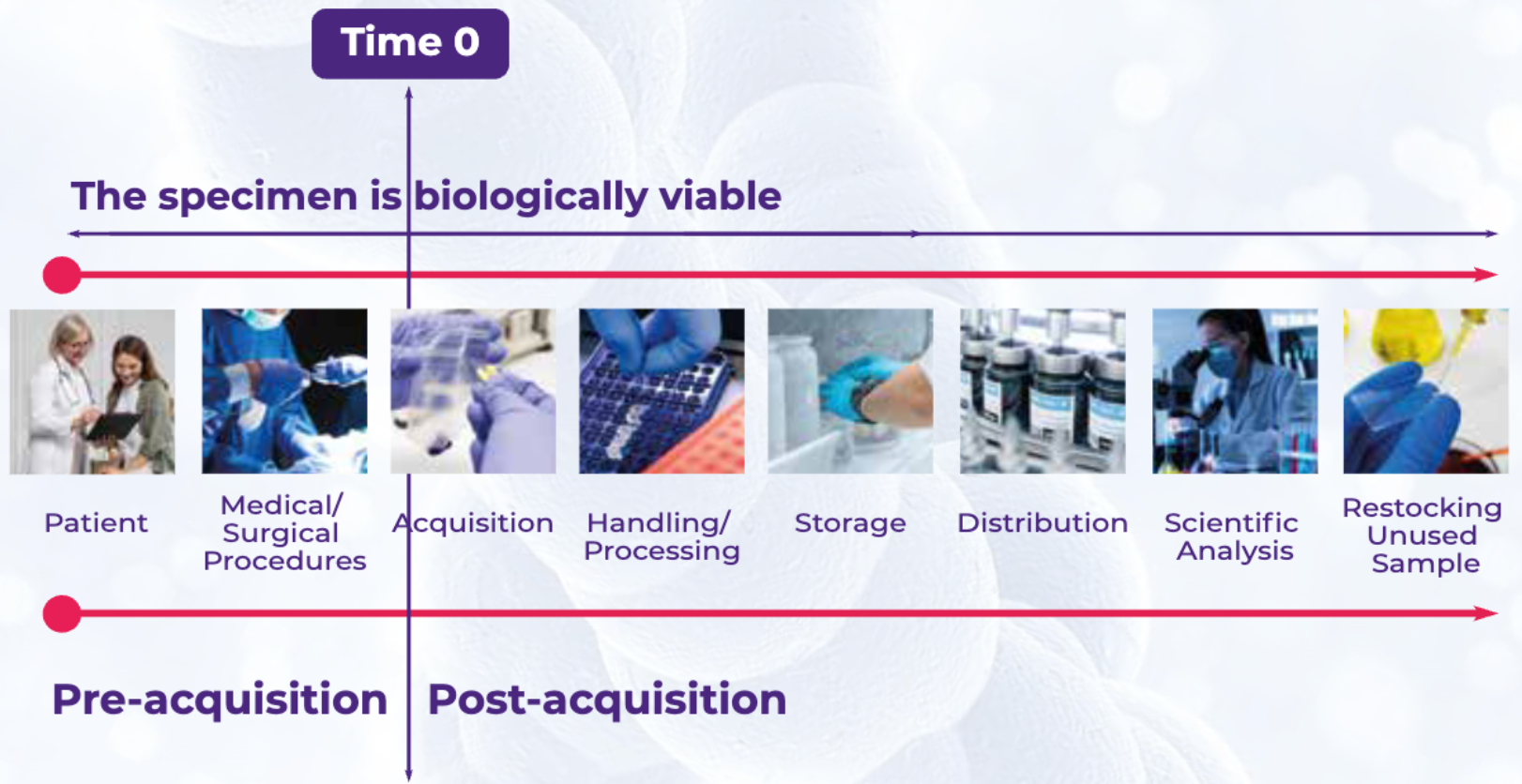
Figure 3 depicts the lifecycle of a biospecimen following acquisition. The method of collection required depends on the type of biospecimen needed (e.g., blood, tissues, cell products, custom-matched sets, etc.). Additionally, biological sample handling and storage procedures for biospecimen collection must be standardized. The Standard PREanalytical Code (SPREC v2.0 and v3.0) is a coding system that reports how variations in biospecimen characteristics can impact downstream results. Visit the Audubon Bioscience blog to learn more about Standardized Preanalytical Coding (SPREC) in tissue biobanking.
Quality Control
The quality control measures in biospecimen collection should be detailed comprehensively from the start of the research protocol. Implementing proper personnel training and documentation practices are two measures that can help maintain sample integrity. There must also be continuous monitoring of temperature, storage conditions, and transportation of biospecimens.
Step 4: Data Documentation and Tracking
Unique Identifiers
A unique identifier is a specific label assigned to each biospecimen to ensure its traceability throughout the procurement process, as well as prevent sample misidentification. This is especially important in the case of personalized medicine because specimen mix-ups will negatively impact patient outcomes and risk the study’s integrity.
Data Integration
The use of unique identifiers enables data integration, which involves the linking of biospecimen data with participant information on secure and standardized databases. Data integration ensures the accountability and quality of the biospecimen while addressing ethical concerns related to participant privacy.
Step 5: Storage and Prevention
Storage Facilities
Biobanking storage facilities today employ advanced technologies in strict compliance with industry preservation standards to maintain long-term biospecimen viability. Their features include controlled environments, secure specimen management systems, as well as routine quality monitoring.
Long-Term Preservation
Long-term preservation in biorepositories maintains biospecimen quality to allow for the collection of longitudinal data on disease progression and treatment efficacy. In addition to developing standardized operating procedures to maintain consistency in storage conditions, cryopreservation can also be considered, especially for cell and tissue samples, such as tumor tissue samples, which otherwise degrade over time.
Step 6: Distribution and Sharing
Request Processing
Request processing involves receiving, reviewing, and approving requests for biospecimens to ensure they are made available to researchers. This process should include accessible communication platforms, timely responses, and clear predefined approval criteria. Prioritizing requests based on their potential societal and research impact may also help guide decisions and optimize the distribution of these valuable biological materials.
Collaborative Platforms
Biobanking trends include the increasing use of collaborative platforms, which help increase efficiency in request processing and facilitate partnerships in biobank research while protecting patient privacy. They also provide a shared platform on which researchers, clinicians, and other stakeholders can communicate and collaborate, allowing for more comprehensive findings.
Step 7: Reporting and Publication
Data Analysis
Researchers must employ robust statistical techniques that are appropriately suited to the type of data to avoid jeopardizing the validity of their results. In this regard, collaborations with statisticians can be highly valuable because they can provide expert guidance on the choice of analytical methods, as well as their execution and interpretation.
Publication Ethics
Publication ethics are a set of guidelines applied in the publication of scientific and academic literature to promote quality, credibility, and trust in the global research community. These guidelines encourage transparency when reporting findings to discourage data misrepresentation and improve the peer review process. Ultimately, a strong commitment by researchers to ethical practices and good research conduct helps protect credibility within the scientific community.
Conclusion
In conclusion, biospecimen procurement is a complex process that plays a key role in driving biobanking trends and innovation. Developing a greater understanding of it is crucial for scientists and healthcare stakeholders, given its impact on personalized medicine advancement and therapeutic development. Each step, from planning and collection to reporting and publication, must be conducted with precision to ensure quality results, good research conduct, and optimal biosample use. By successfully navigating this intricate process, researchers influence the reliability of patient outcomes, and in turn, the progress of scientific research.
Learn more here about how our customized biospecimen procurement approach is supporting biomedical research and visit our website to explore our collection of matched biospecimen sets for your next project. Contact an Audubon Bioscience representative today for more information!
References
- Tarling TE, Byrne JA, Watson PH. The Availability of Human Biospecimens to Support Biomarker Research. Biomarker Insights. 2022;17:117727192210917. doi:https://doi.org/10.1177/11772719221091750
- Audubon Bioscience. Advancing Biospecimen Procurement with Emerging Technology. High-Quality Human Biospecimens. Published October 31, 2023. Accessed December 20, 2023. https://audubonbio.com/blog/advancing-biospecimen-procurement-with-emerging-technologies/
- Gartel G, Scuderi H, Servay C. Implementation of Common Rule Changes to the Informed Consent Form: A Research Staff and Institutional Review Board Collaboration. Ochsner Journal. 2020;20(1):76-80. doi:https://doi.org/10.31486/toj.19.0080
- Weil CJ. Ethical, Legal, and Policy Issues Surrounding Biospecimen Research Conducted or Supported in the USA. Biopreservation and Biobanking. Published online February 9, 2022. doi:https://doi.org/10.1089/bio.2021.0094
- Biospecimen and Data Research – Institutional Review Board. Accessed December 20, 2023. https://irb.wisc.edu/manual/investigator-manual/conducting-human-participant-research/different-types-of-research/biospecimen-and-data-research/
- ISBER. isber.org. Accessed December 20, 2023. https://www.isber.org/general/custom.asp?page=SPREC
- João D, Cardoso S, Monteiro P, Leal C, Bartosch C. Short tandem repeat analysis: a practical tool to identify specimen mix-ups in the pathology laboratory. Virchows Archiv: An International Journal of Pathology. 2023;483(4):549-554. doi:https://doi.org/10.1007/s00428-023-03578-7
- Asiimwe R, Lam S, Leung S, et al. From biobank and data silos into a data commons: convergence to support translational medicine. Journal of Translational Medicine. 2021;19(1). doi:https://doi.org/10.1186/s12967-021-03147-z
- Sanderson-November M, Silver S, Hooker V, Schmelz M. Biorepository best practices for research and clinical investigations. Contemporary Clinical Trials. Published online September 2021:106572. doi:https://doi.org/10.1016/j.cct.2021.106572
- Durant TJS, Gong G, Price N, Schulz WL. Bridging the Collaboration Gap: Real-time Identification of Clinical Specimens for Biomedical Research. Journal of Pathology Informatics. 2020;11(1):14. doi:https://doi.org/10.4103/jpi.jpi_15_20
- Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, Lakey JR. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplantation. 2021;30:096368972199961. doi:https://doi.org/10.1177/0963689721999617
- BioLINCC. The BioLINCC Handbook a Guide to the NHLBI Biologic Specimen and Data Repositories. Accessed December 20, 2023. https://biolincc.nhlbi.nih.gov/media/guidelines/handbook.pdf?link_time=2023-05-02_21:58:03.239908
- Pan P. Ethics in research and publication. Journal of Indian Association of Pediatric Surgeons. 2020;25(6):349. doi:https://doi.org/10.4103/jiaps.jiaps_219_19
- Kretser A, Murphy D, Bertuzzi S, et al. Scientific Integrity Principles and Best Practices: Recommendations from a Scientific Integrity Consortium. Science and Engineering Ethics. 2019;25(2):327-355. doi:https://doi.org/10.1007/s11948-019-00094-3
- Iudchenko A. Medical Staff’s Role in Biospecimen Procurement. High-Quality Human Biospecimens | Audubon Bioscience. Published June 17, 2021. Accessed December 20, 2023. https://audubonbio.com/blog/medical-staffs-role-in-biospecimen-procurement/
- Institutional Review Board | Human Research Protection Program | University Hospitals | Cleveland, OH | University Hospitals. www.uhhospitals.org. https://www.uhhospitals.org/uh-research/for-researchers/research-and-clinical-trials/core-offices/hrpp/irb-process-and-schedule
- Moore H. The NCI Biospecimen Research Network. Biotechnic & Histochemistry. 2011;87(1):18-23. doi:https://doi.org/10.3109/10520295.2011.591833

