Medical Staff’s Role in Biospecimen Procurement

Medical staff's role in biospecimen procurement is a key factor for the development and conduct of this field. Yet, it often ramains largely misread.
Biospecimens are being largely recognized as a crucial element for developing biomarker-based, targeted therapies and diagnostics. While essential to biomedical research advancement, biospecimen procurement is also a complex process involving the well-coordinated efforts of various players 1,2. This multiplayer nature of biobanking creates a certain degree of disconnection and often leaves the different parties with only a partial vision and understanding of the entire biospecimen journey.
At Audubon, we have made it our mission to change that. We strongly believe that achieving a productive and seamless workflow requires a deep understanding and awareness of the biobanking process by all involved parties. For this, we have initiated a series of articles starting with an overview of the biospecimen procurement environment. We continue by turning the spotlight to different areas and professions within the field.
In this article, we look deeper into the crucial role medical staff play within the process and some of the key aspects they oversee.
Biosamples journey to becoming life-saving therapies
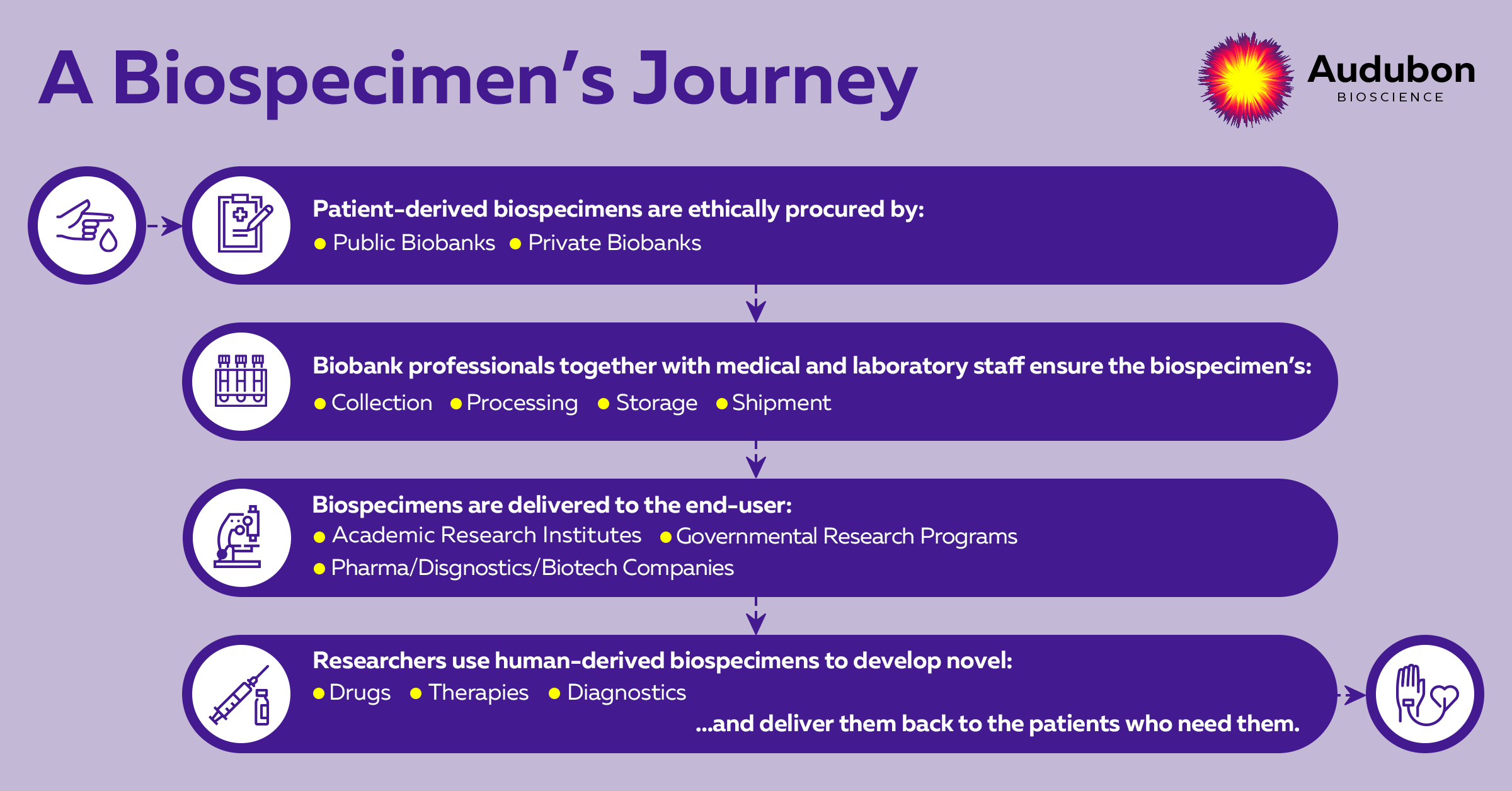
To efficiently convey the vital role of medical staff during biospecimen collection, we need to look at the journey each biospecimen undergoes (Graph 1).
Each step from this graph involves many levels and is a complex process on its own. The journey takes place in between two patients - a donor patient and a patient receiving the treatment. With today's drug and therapy development timelines, it is quite unlikely that a donor patient can benefit from the therapy he contributed to. And this is one main reason why efficient biospecimen procurement for faster drug development is crucial.
To achieve this goal, biobanking teams work closely with the medical staff to ensure the ethical and correct collection of the biosamples, which will be later delivered to their final destination: academic, private, or governmental research groups. Scientists need biospecimens mainly for the early preclinical stages of drug development or biomarkers discovery, and their final goal is to support innovative life-saving solutions for patients in need.
Can all biosamples support biomedical research?
Although biospecimens are an essential source for biomedical research, it is crucial to outline that only samples replying to a stringent list of criteria can be a powerful source for translational research and support unbiased result generation. The first and foremost criteria that specimens need to comply with is to be ethically procured and de-identified to protect patient's privacy.
Biospecimen collection uniformity is of great importance for successful preclinical analysis, which will become the supporting ground for the clinical trial phases of a test drug or therapy. To ensure the uniformity of a collection, each project defines inclusion and exclusion criteria such as demographic data, genetic background, or treatment history. Only patients corresponding to these criteria can donate biospecimens for the study.
A growing body of evidence shows that even small changes in collection, transportation, or storage conditions can seriously alter sample integrity and molecular profiles 3,4,5. Therefore, each study also protects the uniformity of the samples by outlining precise protocols and guidelines for the preanalytical phase of a biosample's life, i.e., the time between collection and analysis. Therefore, all biospecimens must be collected, transported, processed, and stored following the study's SOP.
And last but not least, a sample's fullest potential is revealed by the accompanying clinical data. Any biospecimen with lost or incomplete medical data loses its potential to support biomedical research.
Ensuring that biospecimens meet all of the criteria mentioned above is not an easy task. The key to success in it is to work with qualified and devoted medical and laboratory staff.
What is medical staff's role in biospecimen procurement?
Clinicians are key figures for making biosamples available for research. As they are the point of contact with the patient, they are the ones responsible for rigorously informing them about the act of donating and presenting them to the donation consent form. Doctors are also the ones ensuring patients are not harmed, and their treatment and diagnosis are not affected by the donation. It is again the medical staff that de-identifies the patient's data and protects their privacy.
Medical doctor's expert opinion is a leading point even before a collection has been initiated and the project is still at the design phase. Their expert opinion is vital when biobanking teams are validating a study's feasibility and timelines of execution. Clinicians also make sure patients meet all inclusion and exclusion criteria.
With the advancement of our understanding of disease mechanisms, these are becoming relatively narrow, and in search of better personalized treatments, scientists are looking for very specific pools of patients. This high specificity leads to collections often being conducted at multiple sites around the globe. Although this increases the genetic and ethnic diversity of studies, it also requires full respect for the collection's SOP.
And last but not least medical staff are also overseeing the process of collecting extensive patient medical and demographic data, increasing further the value of the collected sample for downstream research purposes.

Audubon appreciates its medical partners!
At Audubon, we know that success is impossible without our medical staff partners. We greatly appreciate their daily efforts first in treating and helping patients and then in supporting new therapeutics development and protecting patient’s safety throughout the process.
You can learn more about the medical environments we collaborate with at our Geographical Locations page, where we outline our clinical sites network. If you are curious to know more about the essential role clinicians and doctors play in biospecimen procurement, check out our dedicated webpage or contact us. We will be happy to answer your questions!
References:
- Zatloukal, K. & Hainaut, P. Human tissue biobanks as instruments for drug discovery and development: impact on personalized medicine. Biomark. Med. 4, 895–903 (2010).
- Kapp, M. B. Ethical and legal issues in research involving human subjects: Do you want a piece of me? J. Clin. Pathol. 59, 335–339 (2006).
- Müller, H. et al. Biobanks for life sciences and personalized medicine: importance of standardization, biosafety, biosecurity, and data management. Curr. Opin. Biotechnol. 65, 45–51 (2020).
- Meddeb, R., Pisareva, E. & Thierry, A. R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 65, 623–633 (2019).
- Gottfried-Blackmore, A. et al. Effects of processing conditions on stability of immune analytes in human blood. Sci. Rep. 10, 17328 (2020).

