The Importance of Biobanking in Molecular Diagnostics & Drug Discovery

Biobanking is a rapidly evolving field that has become central to driving advancements in preclinical research and supporting drug discovery. Biobanks are repositories containing large collections of biological human samples, including tissue samples, blood, and DNA.1 These repositories provide scientists access to a vast array of biosamples for research, which are used to gain further insight into disease mechanisms and improve patient outcomes.1 However, despite its significant contributions to biomedical research, biobanking has not yet reached its full potential.2 Certain challenges, such as the ethical and legal implications of biobanking, as well as technical and logistical limitations faced by researchers have to be addressed.
In this article, we dive into the role of biobanking in molecular diagnostics and preclinical research, explore what hurdles researchers are currently facing, and potential solutions on the horizon that could mitigate these challenges. Read on to learn more!
An Overview of Molecular Diagnostics
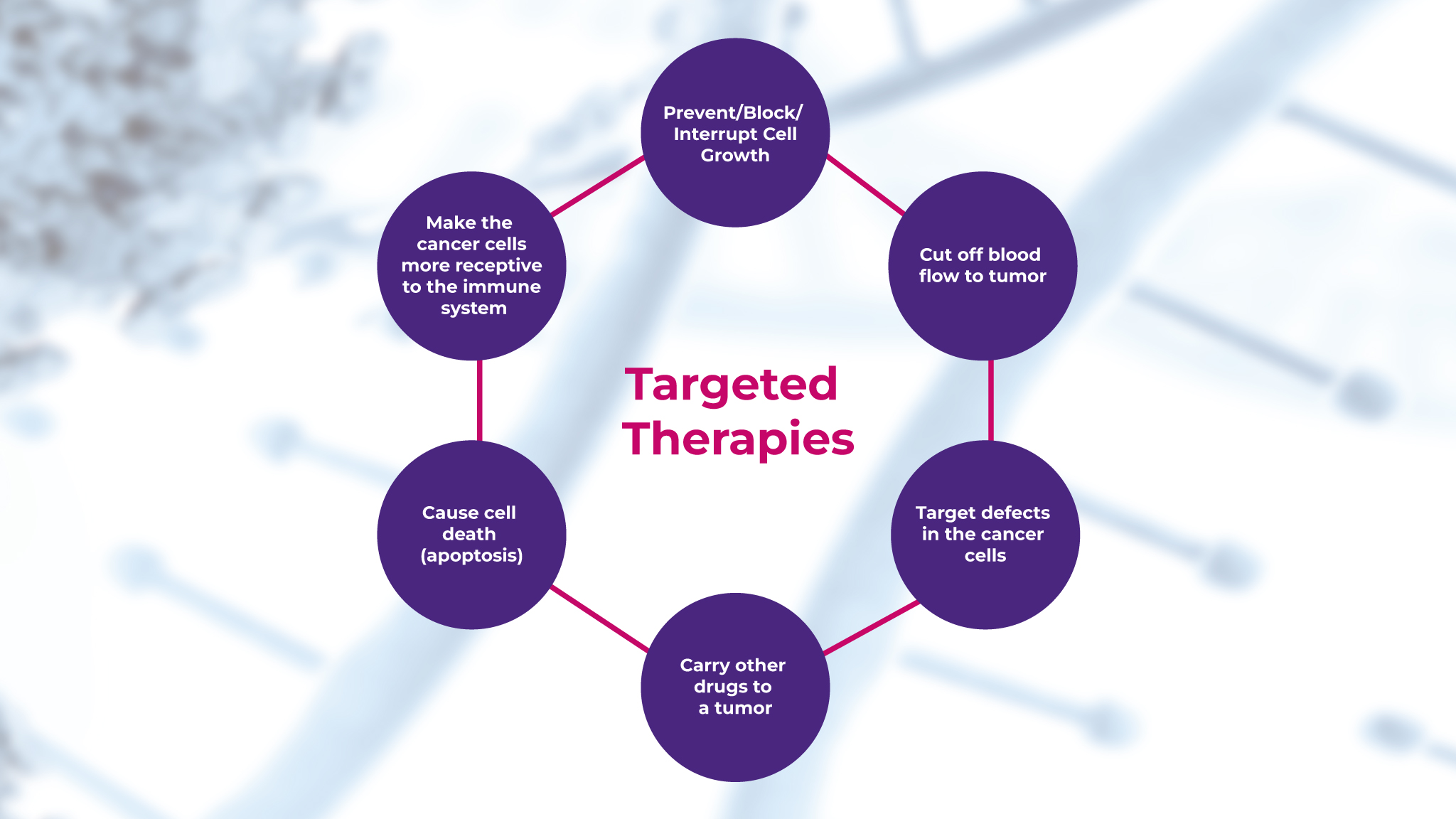
Molecular diagnostics consists of an array of techniques applied for the analysis of DNA, RNA, and proteins at the molecular level to detect and measure genetic mutations or alterations that may predispose certain diseases.3 In the field of oncology, traditional treatments like chemotherapy and radiation therapy are typically associated with significant side effects.4 However, with the advent of targeted therapies, which are based on understanding the genetic makeup of the tumor tissue samples, cancer treatment has become more personalized and effective, as shown in Figure 1.5
Unlike traditional therapies, these target specific genetic mutations in the tumor, thereby minimizing the damage done to healthy cells and improving patient outcomes.5 Read this 2023 review here about the current landscape of targeted cancer therapies.
Why is Biobanking Important in Molecular Diagnostics?
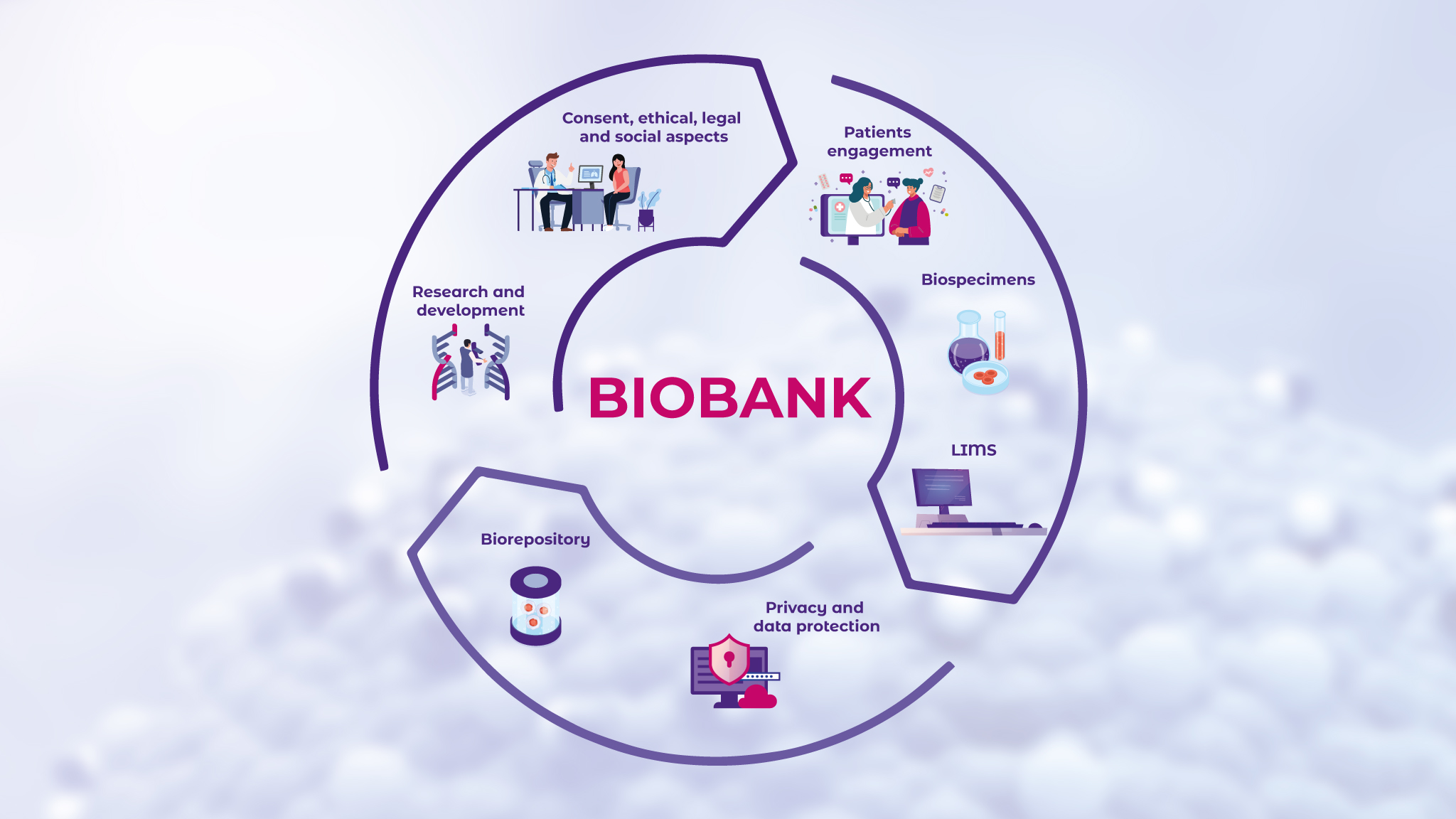
Biobanking, the practice of biosample collection plays a pivotal role in molecular diagnostics and is depicted generally in Figure 2.7 By analyzing biobanked samples, scientists can identify key genetic mutations and other molecular alterations that drive disease progression.8 Other contributions that biobanks have made in advancing the field of biomedical research are biomarker discovery and the development of diagnostic tests.9,10
Using the resources such repositories provide, researchers can identify and validate new disease biomarkers, which are measurable indicators of biological conditions or responses.11 In clinical practice, biomarkers and diagnostic tests serve as essential tools in early disease detection, disease progression monitoring, and treatment effectiveness evaluation.
Biobanking Contributions to Drug Discovery
Identification of Potential Drug Targets
Samples provided by biobanks can be analyzed extensively to identify potential drug targets. In the case of hereditary breast cancer, genomic data from biobanked cancer tissue samples led to the development of the trastuzumab antibody, Herceptin. Since its inception, Herceptin has become a well-known drug for effectively treating patients with certain forms of breast cancer.13
Biomarker Validation
Biobanks provide biopharmaceutical companies with the required sample diversity and volume to confirm the predictive value of identified biomarkers. Large biobanks provide tissue samples collected from cancer patients, aiding in translational research. The resulting biomarker discovery and validation could potentially identify patients who are ideal candidates for immunochemotherapy.14
Preclinical Testing
Biobanks also offer an invaluable source of human biological samples that can be used to assess drug safety and efficacy before clinical trials. For example, tumor organoid biobanks have served as a preclinical model for screening anticancer drugs, enabling researchers to evaluate their effects on human cancer tissue samples directly before clinical trials.15
Factors Limiting Availability of Well-Annotated Biospecimens for Research Use
Despite their importance, the availability of well-annotated biospecimens is often limited due to several factors.
Regulatory Challenges
One of the major hurdles in research biospecimen acquisition is navigating the stringent regulatory environment.16 For example, although informed consent is an essential pillar of good research conduct and ethical standards in biobanking, samples are limited for use in the specific project that the donor consented to. The globalization of research also adds hurdles of complying with multiple local regulatory authorities while utilizing biobanking samples.16
However, Audubon's extensive experience in the regulatory field enables researchers and biopharmaceutical companies to efficiently secure the necessary ethical approvals and informed consent. They can ensure any provided biospecimen for molecular diagnostics or other research use are ethically sourced and compliant with all regulatory requirements.
Technical Difficulties
The biospecimen procurement process requires not only specialized equipment but also personnel with highly specialized expertise in handling biosamples for research. Moreover, without robust data management systems, biobanked samples may be poorly annotated, missing key details of relevant clinical data, such as the donor’s health status, disease progression, and treatment response.16
Audubon Bioscience excels in this aspect by fielding a highly trained team of professionals well-versed in collecting and providing well-annotated biospecimens. Their meticulous processes ensure that each specimen's molecular integrity remains intact throughout.
Financial Constraints
The costs involved in biosample collection, processing, storage, and distribution can also be prohibitive. These expenditures include not only the direct costs of equipment and personnel but also indirect costs such as infrastructure and administrative expenses.16
Audubon Bioscience addresses this issue by offering a cost-effective option for obtaining high-quality specimens, including formalin-fixed paraffin-embedded (FFPE) tissue samples, with an array of services spanning from collection to distribution. This model allows researchers to obtain such resources and focus on advancing various fields, such as precision medicine and preventative genomics, instead of investing heavily in overhead costs. Explore our catalog of diverse high-quality human biospecimens on the Audubon website here.
Why Quality Assurance and Standards are Crucial for Good Research Conduct
In biobanking, the stringent process of quality assurance (QA) entails rigorous testing, monitoring, and documentation to maintain sample integrity and reduce variability.17 These robust QA measures ensure the specimens stored are of the highest quality, thus directly improving the validity and reproducibility of biomedical research conducted on these samples.
Furthermore, because human biospecimens often come with personal identifiers, biobanks must adhere to strict ethical practices to protect donors’ rights and privacy.7 These involve upholding good research conduct with informed consent and confidentiality to foster greater public trust in the use of biobanking.17 Audubon Bioscience, a renowned biobank, is one of many institutions that have built a trustworthy reputation by upholding the highest QA and ethical standards in their modern specimen collection process. Learn more here about the step-by-step process with which Audubon consistently procures quality well-annotated biospecimens.
Exploring Key Advancements in Biobanking Technologies
Several technological advancements in biobanking are rapidly revolutionizing the collection, storage, and utilization of biospecimens for molecular diagnostics and other research use.
Automation in Biobanking Technologies
Automated systems have not only streamlined the process of modern specimen collection and storage but have also minimized human errors, thereby ensuring improved accuracy and consistency in the biospecimen procurement process.18 By enabling high-throughput processing, these automated solutions are providing an efficient alternative to traditional sample handling procedures, conveniently reducing time, resources, and manual labor required from researchers.
Advances in Cryopreservation Techniques
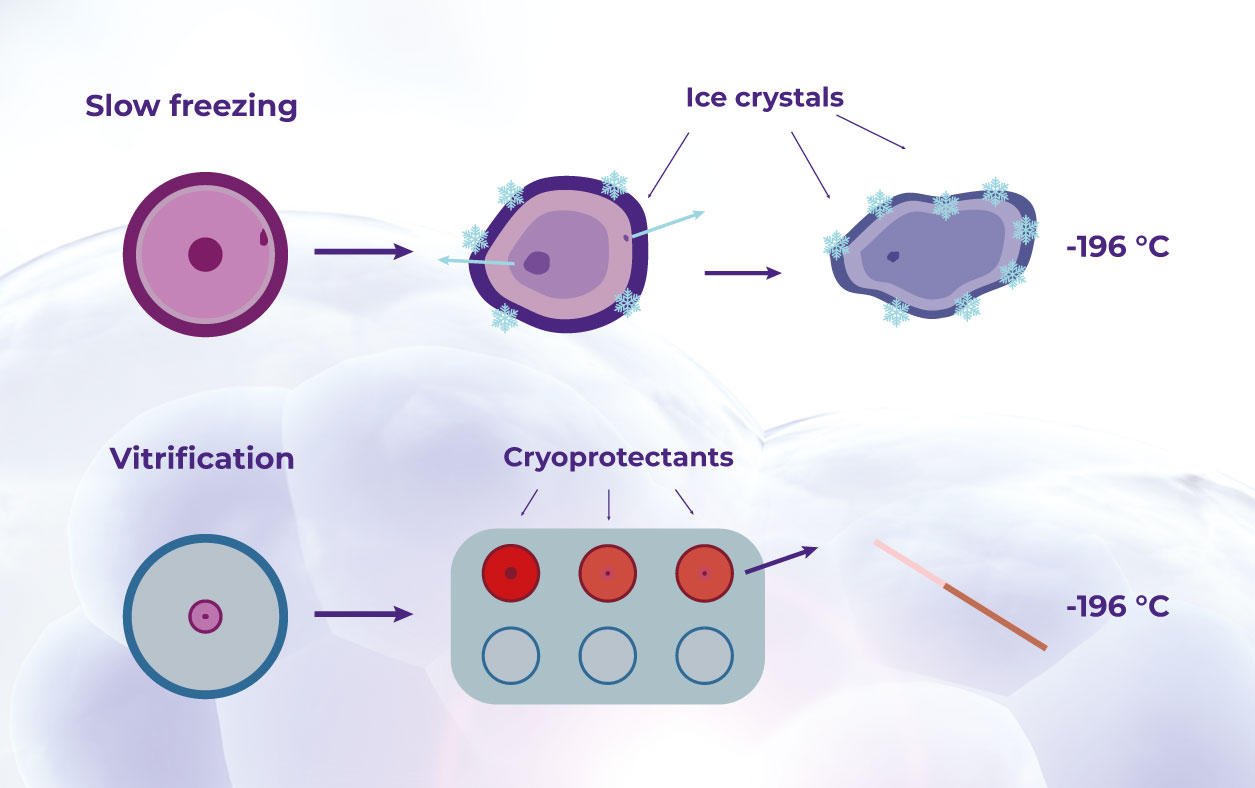
The effectiveness of cryopreservation techniques is crucial in preserving the biological characteristics of the samples, which, in turn, determines their usefulness in research. Advancements such as vitrification and high-subzero, now allow for long-term storage of samples at ultra-low temperatures.19 With these, biobanks can store biospecimens for extended periods while effectively minimizing quality degradation and any cellular damage.19
Data Management Systems in Biobanking
Data management systems allow for the efficient tracking and cataloging of stored samples, as well as the management of associated data. With the advent of cloud-based data management systems, biobanks can now manage and access their data remotely, offering greater flexibility, efficiency, and accessibility for life science researchers and biopharmaceutical companies.21 The implementation of this technology also facilitates strict compliance with regulatory requirements for data privacy and security, enhancing the credibility of biobanks.
Contributions of Companies Like Audubon Bioscience
As an industry leader in research biospecimen acquisition, Audubon Bioscience could provide invaluable services as biopharmaceutical companies increasingly incorporate artificial intelligence to drive drug development.22 By supplying high-quality, well-characterized biospecimens, Audubon could support these companies in training their machine learning models, thereby facilitating the development of more precise and effective drugs.
Conclusion
In conclusion, biobanking is playing a pivotal role in advancing molecular diagnostics and drug discovery. Although biospecimen procurement processes provide invaluable insights for scientists, the full potential of biobanking still has yet to be realized. Challenges such as ethical and legal issues, technical limitations, and financial constraints need to be addressed. However, several technological advancements and strict QA measures are helping mitigate these challenges, paving a path for improved processes in biobanking. Leading the way are companies like Audubon Bioscience, with their consistent, reliable commitment to ethical sourcing and high-quality specimens. As molecular diagnostics and drug discovery progresses forward, the role of biobanks will only increase, making them an essential component in medical research.
Visit our website to explore our collection of matched biospecimen sets and other biospecimen solutions for your next project. Contact an Audubon Bioscience representative today for more information!
References
- Malsagova K, Kopylov A, Stepanov A, et al. Biobanks—A Platform for Scientific and Biomedical Research. Diagnostics. 2020;10(7):485. doi:https://doi.org/10.3390/diagnostics10070485
- Sulaieva ON, Artamonova O, Dudin O, et al. Ethical navigation of biobanking establishment in Ukraine: learning from the experience of developing countries. Journal of Medical Ethics. Published online November 9, 2023. doi:https://doi.org/10.1136/jme-2023-109129
- Liu Q, Jin X, Cheng J, Zhou H, Zhang Y, Dai Y. Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens (Review). Molecular Medicine Reports. 2023;27(5). doi:https://doi.org/10.3892/mmr.2023.12991
- Chemotherapy Side Effects. www.cancer.org. Accessed February 15, 2024. https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/chemotherapy-side-effects
- What is Targeted Therapy? Cancer.net. Published December 19, 2013. Accessed February 15, 2024. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/personalized-and-targeted-therapies/what-targeted-therapy
- Lung Cancer Targeted Therapies. www.medschool.lsuhsc.edu. Accessed February 15, 2024. https://www.medschool.lsuhsc.edu/lungcancer/targeted_therapies.aspx
- Annaratone L, De Palma G, Bonizzi G, et al. Basic principles of biobanking: from biological samples to precision medicine for patients. Virchows Archiv. 2021;479(2):233-246. doi:https://doi.org/10.1007/s00428-021-03151-0
- Lazareva TE, Barbitoff YA, Changalidis AI, et al. Biobanking as a Tool for Genomic Research: From Allele Frequencies to Cross-Ancestry Association Studies. Journal of Personalized Medicine. 2022;12(12):2040. doi:https://doi.org/10.3390/jpm12122040
- Matzke LA, Watson PH. Biobanking for Cancer Biomarker Research: Issues and Solutions. Biomarker Insights. 2020;15:117727192096552. doi:https://doi.org/10.1177/1177271920965522
- Ongarello S, Fernández Suárez M, Betsou F. The DxConnect Virtual Biobank connects diagnostic researchers to clinical samples. Nature Biotechnology. 2021;40(1):18-19. doi:https://doi.org/10.1038/s41587-021-01168-z
- Ahmad A, Imran M, Ahsan H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics. 2023;15(6):1630-1630. doi:https://doi.org/10.3390/pharmaceutics15061630
- Bonizzi G, Capra M, Cassi C, et al. Biobank for Translational Medicine: Standard Operating Procedures for Optimal Sample Management. Journal of Visualized Experiments. 2022;(189). doi:https://doi.org/10.3791/63950
- Coppola L, Cianflone A, Grimaldi AM, et al. Biobanking in health care: evolution and future directions. Journal of Translational Medicine. 2019;17(1). doi:https://doi.org/10.1186/s12967-019-1922-3
- Lommen K, Odeh S, de Theije CC, Smits KM. Biobanking in Molecular Biomarker Research for the Early Detection of Cancer. Cancers. 2020;12(4). doi:https://doi.org/10.3390/cancers12040776
- Botti G, Di Bonito M, Cantile M. Organoid biobanks as a new tool for pre-clinical validation of candidate drug efficacy and safety. International Journal of Physiology, Pathophysiology and Pharmacology. 2021;13(1):17-21. Accessed July 9, 2021. https://pubmed.ncbi.nlm.nih.gov/33815668/
- Longo S. Invisible Markets: The Chilling Truth of the Black Market Biospecimen Business. Scientist.com. Published July 25, 2023. Accessed February 15, 2024. https://crex.nih.gov/blog/2023/07/25/invisible-markets-the-chilling-truth-of-the-black-market-biospecimen-business
- QA Process: A Complete Guide to QA Stages, Steps, & Tools. Testsigma Blog. Published July 27, 2023. https://testsigma.com/guides/qa-process/
- Fuchs YF, Brunner J, Weigelt M, et al. Next Generation Biobanking: Employing a Robotic System for Automated Mononuclear Cell Isolation. 2023;21(1):106-110. doi:https://doi.org/10.1089/bio.2021.0181
- Taylor Michael J, Weegman Bradley P, Baicu Simona C, Giwa Sebastian E. New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfusion Medicine and Hemotherapy. 2019;46(3):197-215. doi:https://doi.org/10.1159/000499453
- What is IVF with vitrified eggs and what are the results? inviTRA. Published May 10, 2021. https://www.invitra.com/en/what-is-ivf-with-vitrified-eggs-and-what-are-the-results/
- Kinkorová J, Topolčan O. Biobanks in the era of big data: objectives, challenges, perspectives, and innovations for predictive, preventive, and personalised medicine. EPMA Journal. 2020;11(3):333-341. doi:https://doi.org/10.1007/s13167-020-00213-2
- Licholai G. AI Poised To Revolutionize Drug Development. Forbes. Accessed February 15, 2024. https://www.forbes.com/sites/greglicholai/2023/07/13/ai-poised-to-revolutionize-drug-development/?sh=4369dcf27ca4

