How Biomarker Discovery and Validation Supports Targeted Cancer Therapies
Biomarkers are essential tools in oncology research, allowing for early detection, diagnosis, and personalized cancer treatment.1 For this reason, driving innovation in biomarker discovery and validation is critical for developing targeted therapies to improve clinical outcomes. Here, we discuss the concept of biomarkers in the field of oncology and why they have been indispensable to the development of targeted cancer therapies.
Introducing Biomarker Discovery and Validation
Biomarkers are objective measurable indicators of normal or pathogenic biological processes. Originating from genetic materials, proteins, lipids, or metabolites, biomarkers can be used to diagnose, monitor, and predict a patient's response to treatment 1. Their role is especially crucial in the field of cancer research, where they often indicate the presence of a tumor, as well as its location and disease stage in patients 2.
Cancer biomarkers also help predict the outcome of treatment, monitor treatment response, and guide clinical decisions. Some notable examples of biomarkers include those used to guide targeted therapy in breast cancer, such as the breast cancer susceptibility genes (BRCA1/2) and the human epidermal growth factor receptor 2 (HER2) 1,3. Therefore, considering their value in clinical oncology, successful biomarker discovery, and validation are key steps in the development of effective targeted cancer therapies.
The Importance of Targeted Cancer Therapies
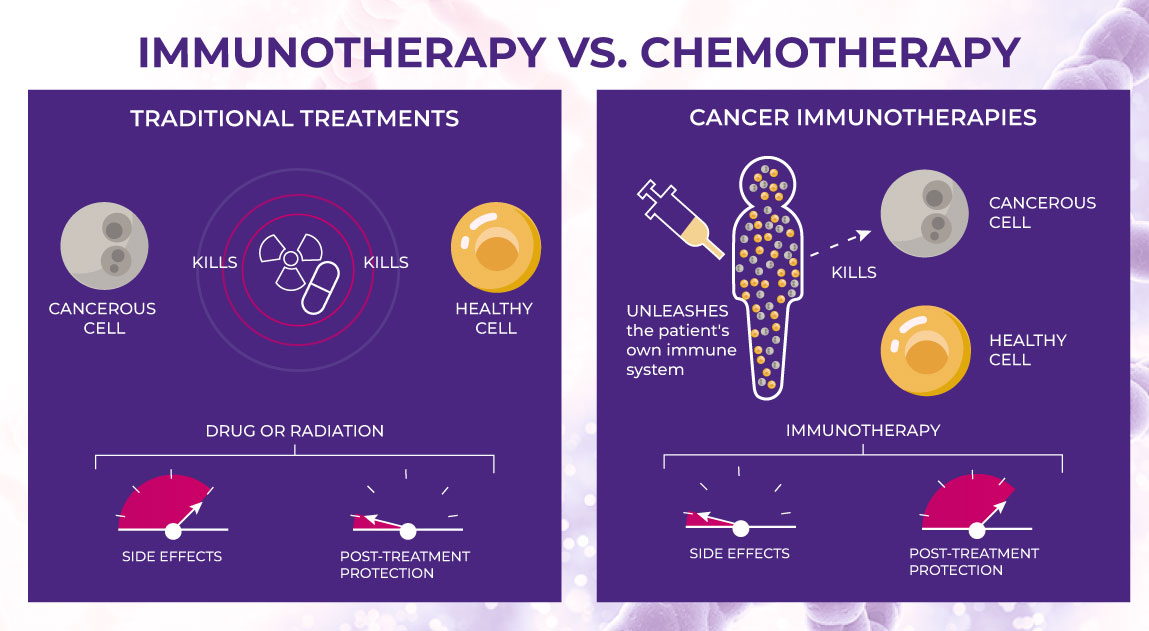
Conventional treatments such as chemotherapy and radiation therapy have long been used to treat cancers; however, because these are not tumor-selective, healthy cells also become damaged during therapy. As a result, patients undergoing such conventional treatments often suffer from severe and long-lasting side effects.4
In contrast, targeted cancer therapies use drugs that are specifically designed to attack cancerous cells without affecting healthy ones, allowing for greater efficacy in conjunction with fewer safety concerns. Furthermore, a hallmark of the challenges faced by oncology scientists and clinicians lies in the disease heterogeneity of cancer, wherein different tumor types will often require different treatments.5
The use of targeted therapies helps account for this because these drugs can be designed based on the unique makeup of a patient’s cancer cells, thereby promoting more effective treatment and better clinical outcomes.
Audubon Bioscience and Personalized Oncology
Because abnormal tumor activity can arise from a variety of genetic mutations, taking a one-size-fits-all approach to oncology drug development is not always appropriate.
At Audubon Bioscience, we recognize the need for patient-centric personalized cancer treatment and are devoted to paving the way forward in this direction with our quality biospecimens. Audubon functions at a global level collecting large, diverse arrays of biospecimens to support the discovery of promising targeted therapeutic drugs, particularly in the pre-clinical phase.
Discovered unique molecular signatures present in patient samples are utilized to design targeted cancer therapies tailored to an individual’s tumor type.6
Read more about the importance of customized biological sample collection in biomarker development in our recent article here.
Technology within the Biomarker Discovery and Validation Process
Biomarker discovery is the first step, involving identifying specific characteristics and markers associated with different cancers. Once potential biomarkers are identified, they need to be validated in pre-clinical studies to determine their sensitivity, specificity, and reproducibility. After pre-clinical validation, these markers undergo clinical validation, where they are evaluated in large, well-defined clinical trials to determine their effectiveness in diagnosing and predicting treatment outcomes. If cancer biomarkers are found to be effective in clinical trials, they can be used to develop new drugs or repurpose existing drugs for specific cancers.7
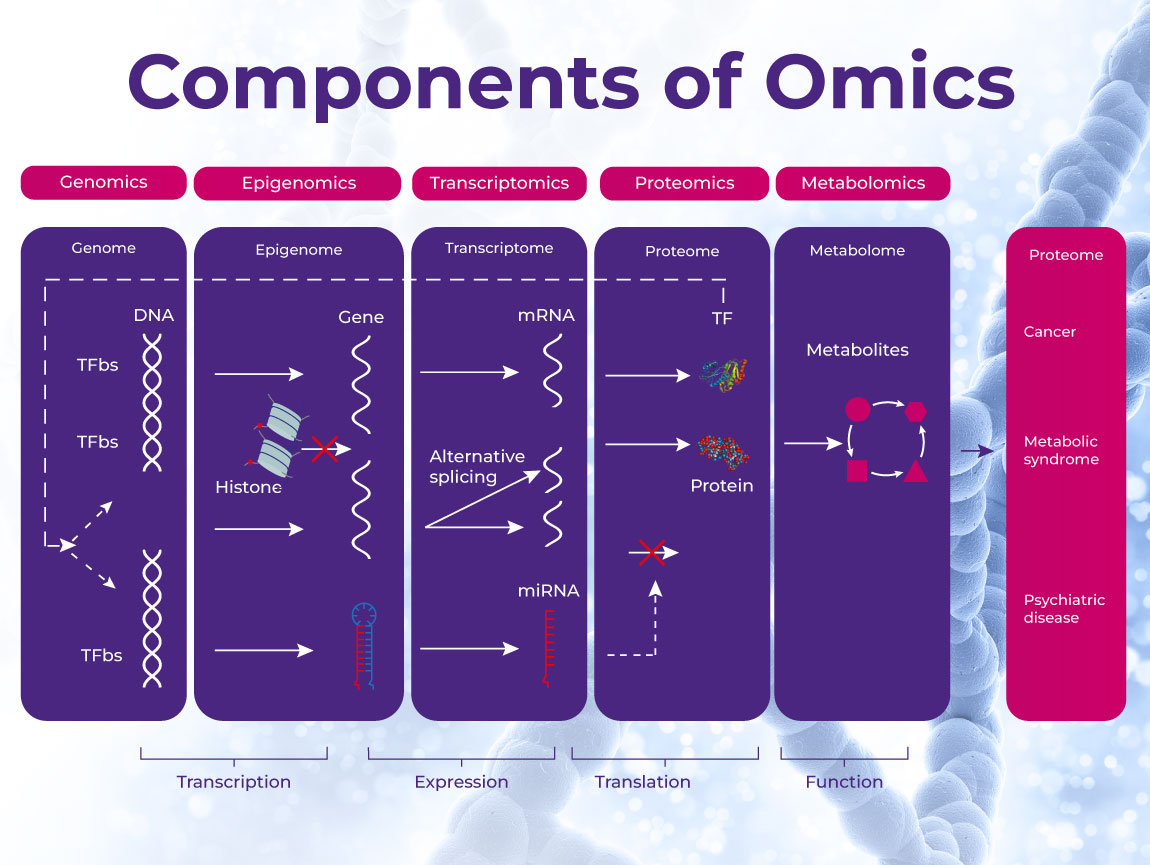
During this process, techniques such as genomics, proteomics, and metabolomics are commonly applied to identify and validate biomarkers. These high-throughput screening methods are used to study an organism’s entire cellular profile of either DNA, proteins, or metabolic pathways, respectively, to identify variations that could be associated with certain tumor occurrences.8
Scientists can also employ imaging techniques such as magnetic resonance imaging (MRI), positron emission tomography (PET), and computerized tomography (CT) scans to monitor changes in tissue structure and function in the context of cancerous growth.9
Additionally, liquid biopsy techniques such as circulating tumor DNA and circulating tumor cells have been used to detect cancer-specific biomarkers in blood samples.10
How Targeted Therapies are Enhancing Cancer Care
The development of targeted cancer therapies has revolutionized clinical oncology with the aid of biomarker research and quality biospecimen availability. Personalized immunotherapy is emerging as a promising option in cancer precision medicine and is demonstrating real benefits. Investments in the development of targeted therapies can improve patient outcomes. 11
-
Personalized cancer therapy targets specific molecular changes in cancer cells, based on the genetic signature of the patient's tumor.
-
Immunotherapy works by boosting the body's immune system to attack cancer cells.
-
Precision medicine uses genomic analysis to identify which specific genetic mutations are driving a patient's cancer. Then this information is used to develop targeted therapies that are more effective in treating the patient's cancer.
For example, a recent phase 3 trial published in the New England Journal of Medicine, Eskander et al. (2023 found that patients with advanced endometrial cancer treated additionally with pembrolizumab, a targeted therapy, experienced significantly longer progression-free survival than those treated with chemotherapy alone.12
Pembrolizumab was also the first cancer, treatment approved by the US Food and Drug Administration (FDA) in 2017 to treat solid tumors based on genetic characteristics, rather than their location in the body.13
Furthermore, a 2022 Nature study published on the phase 3 DREAMseq clinical trial highlights combination treatment with the targeted therapies nivolumab and ipilimumab as the first-choice frontline treatment for advanced-stage BRAF-mutant melanoma.14,15
The Future of Biomarkers in Personalized Oncology
The journey towards finding a cure for any disease is often a long and arduous one. However, the discovery of biomarkers has revolutionized the way we approach diagnosis and treatment. For example, tumor-specific exosomes, a type of extracellular vesicle, have recently been studied as a diagnostic and prognostic biomarker, as well as a potential therapeutic vehicle for cancer treatment.16
In addition to personalized medicine, biomarkers can also be used for cancer screening and early detection, which improve patient outcomes and reduce healthcare costs. Currently, the availability of valuable information through electronic health records is enabling the potential implementation of population-based risk-stratified cancer screening programs. That is, multifactorial classification of high- and low-risk individuals can receive personalized cancer risk assessment.17
As our access to the latest technological validation techniques evolves, we can expect greater success in the development of targeted cancer therapies. Additionally, there is a significant need for the availability of high-quality biosamples which could enable greater efficiency in preclinical discovery studies and greater success in clinical cancer trials.
Read more here to learn about how Audubon Biosciences is working to meet this need. By leveraging these advances, scientists can better validate the clinical usefulness of cancer biomarkers and explore their accuracy and reliability in disease screening, early detection, and treatment.
Conclusion
At Audubon Bioscience, we understand the importance of biomarker discovery and validation in the fight against cancer, and our diverse biospecimens play a crucial role in this journey. Our team of experts is always ready to assist you in finding the right biospecimen for your research needs. To learn more, explore our dedicated product pages or reach out to a team member today to request a quote!
References
- Bodaghi A, Fattahi N, Ramazani A. Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon. Published online January 30, 2023:e13323. doi: https://doi.org/10.1016/j.heliyon.2023.e13323
- National Cancer Institute. Biomarker Testing for Cancer Treatment - National Cancer Institute. www.cancer.gov. Published October 5, 2017. Updated December 14, 2021. https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment
- Tomasello G, Gambini D, Petrelli F, et al. Characterization of the HER2 status in BRCA-mutated breast cancer: a single institutional series and systematic review with pooled analysis. ESMO open. 2022;7(4):100531. doi: https://doi.org/10.1016/j.esmoop.2022.10053
- Mayo Clinic Staff. Managing the lingering side effects of cancer treatment. Mayo Clinic. Published November 4, 2022. https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/cancer-survivor/art-20045524
- El-Sayes N, Vito A, Mossman K. Tumor Heterogeneity: A Great Barrier in the Age of Cancer Immunotherapy. Cancers. 2021;13(4):806. doi: https://doi.org/10.3390/cancers13040806
- Diao JA, Wang JK, Chui WF, et al. Human-interpretable image features derived from densely mapped cancer pathology slides predict diverse molecular phenotypes. Nature Communications. 2021;12(1). doi: https://doi.org/10.1038/s41467-021-21896-9
- Davis KD, Aghaeepour N, Ahn AH, et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nature Reviews Neurology. 2020;16(7):381-400. doi: https://doi.org/10.1038/s41582-020-0362-2
- Montaner J, Ramiro L, Simats A, et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nature Reviews Neurology. 2020;16(5):247-264. doi: https://doi.org/10.1038/s41582-020-0350-6
- Hussain S, Mubeen I, Ullah N, et al. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Li C, ed. BioMed Research International. 2022;2022:1-19. doi: https://doi.org/10.1155/2022/5164970
- Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. Journal of Hematology & Oncology. 2022;15(1). doi: https://doi.org/10.1186/s13045-022-01351-y
- Kiyotani K, Toyoshima Y, Nakamura Y. Personalized immunotherapy in cancer precision medicine. Cancer Biology and Medicine. 2021;18(-). doi: https://doi.org/10.20892/j.issn.2095-3941.2021.0032
- Eskander RN, Sill MW, Beffa L, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. New England Journal of Medicine. Published online March 27, 2023. doi: https://doi.org/10.1056/nejmoa2302312
- Developing the First Precision Immunotherapy - NCI. www.cancer.gov. Published September 21, 2017. Accessed June 11, 2023. https://www.cancer.gov/research/areas/treatment/immunotherapy/pembrolizumab-first-precision-immunotherapy#:~:text=In%20May%202017%2C%20the%20Food%20and%20Drug%20Administration
- Killock D. DREAMseq of therapy for BRAF-mutant melanoma. Nature Reviews Clinical Oncology. 2023;20(1):1-1. doi: https://doi.org/10.1038/s41571-022-00708-z
- Atkins MB, Lee SJ, Chmielowski B, et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: The DREAMseq Trial - ECOG-ACRIN EA6134. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. Published online September 27, 2022:101200JCO2201763. doi: https://doi.org/10.1200/JCO.22.01763
- Wang X, Tian L, Lu J, Ng IOL. Exosomes and cancer - Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis. 2022;11(1):1-12. doi: https://doi.org/10.1038/s41389-022-00431-5
- Fitzgerald RC, Antoniou AC, Fruk L, Rosenfeld N. The future of early cancer detection. Nature Medicine. 2022;28(4):666-677. doi: https://doi.org/10.1038/s41591-022-01746-x

