How Customized Biospecimen Procurement Support Biomarkers Research?

While biospecimens have convincingly gained their place as key building blocks for the advancement of biomedical research, their full potential, collections design, and regulatory requirements are often not well understood. As the sophistication of biopharmaceutical research and progress are constantly increasing, so is the complexity of customized biospecimen procurement projects.
Here we present our analysis of the procurement projects we performed in 2020 in order to illustrate the critical role that customized prospective procurement play in the advancement of biomedical research, specifically in the context of biomarkers development for unmet medical needs.
How do we tackle challenges in biospecimen procurement?
Identification of informative and specific biomarkers is at the core of precision medicine and biospecimens are a critical component of their discovery, development, and validation. Many of the current hurdles in precision oncology can be traced back to the biosamples on which research is performed. Therefore, solving customized biospecimen procurement challenges directly addresses a wide range of limitations throughout the research and development pipelines1,2.
In the recent article we described the current challenges associated to the lack of ethnic and geographical diversity in biospecimen collections used in biomarkers research3,4. At Audubon, we tackle this issue by constantly expanding our geographic presence and clinical sites network. But we don’t stop there. Another aspect that strongly affects the quality and usability of biospecimen collections is their fit-for-purpose character. To this end, we work towards offering flexible and custom collection capacities to our research partners. To be able to do so we need to gain a deep understanding of the research trends and how they are reflected in biospecimens procurement requests. We closely analysed the trends in the collections requests we’ve received and performed on yearly-basis. This provided us with great insights and understanding of the current research needs and straightened our preparedness to support our partners.
What does the 2020 collections analysis tell us?
In another recent article, we presented our 2020 collections analysis. A main observation we made was that 25.52% of all our projects were collecting matched biospecimen sets.
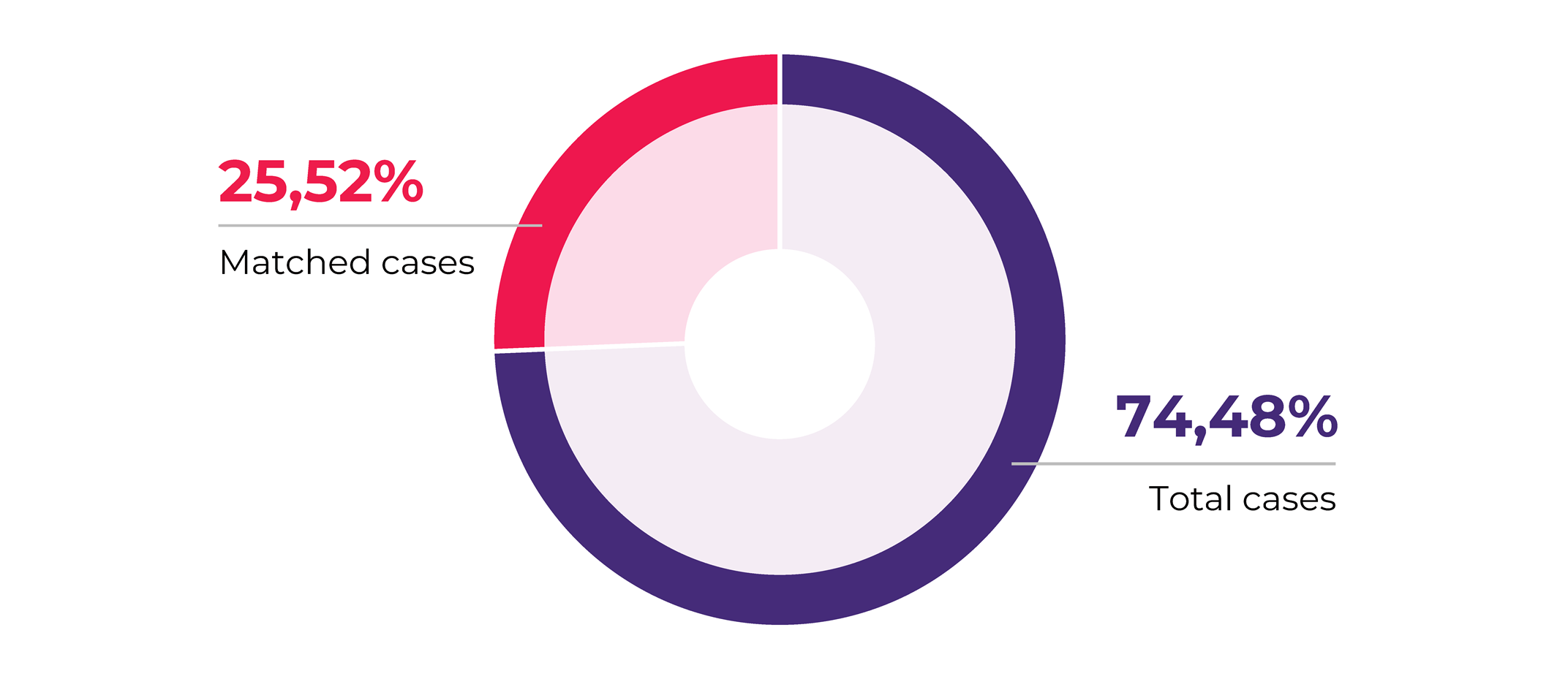
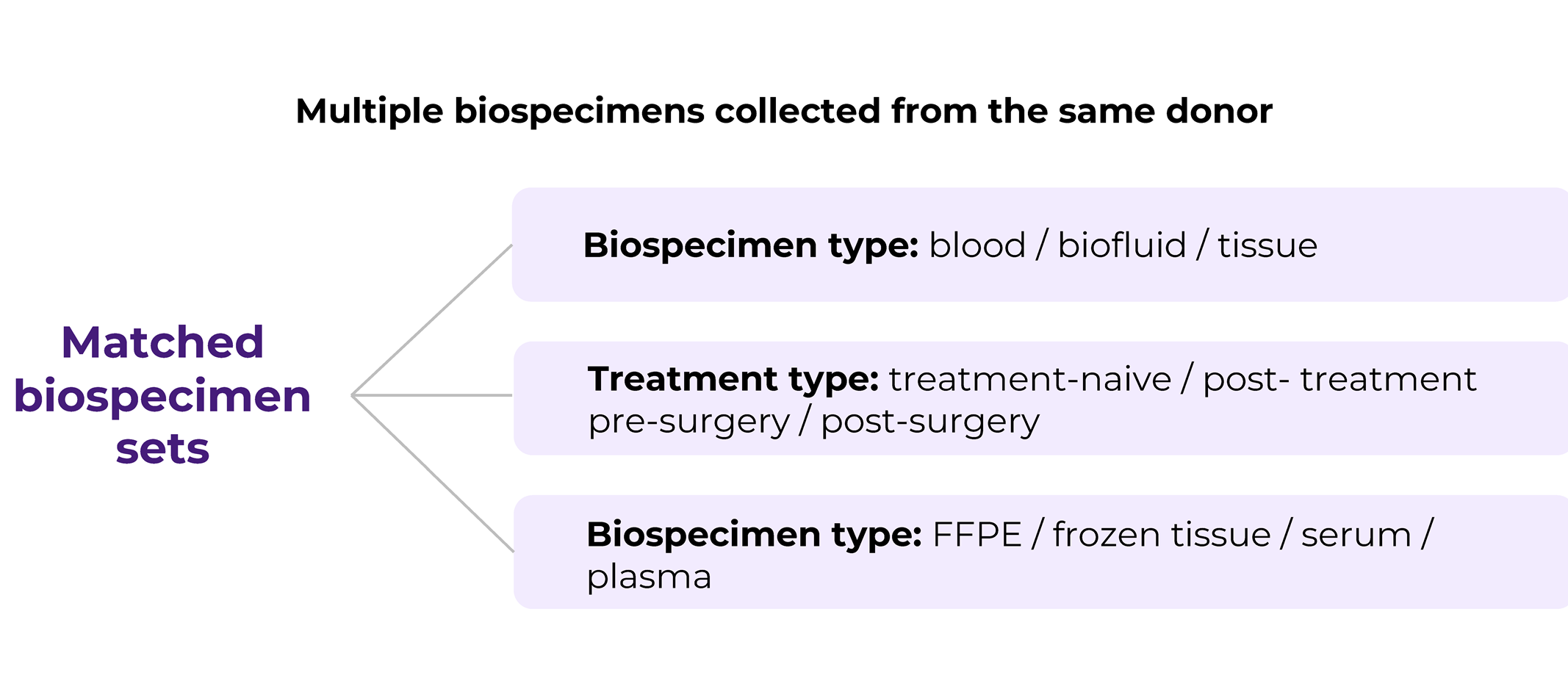
Matched biospecimens are multiple samples collected from the same donor. The matching criteria may vary, for example, it can be different types of biospecimens, i.e., blood and tissue, blood and PBMCs, etc. Or it can the same type of type of biospecimen but collected at different time points and conditions, such as pre/post treatment, pre- or post-surgery. Another option is that it is the same type of tissue (solid tumor tissue, blood) but stored/processed in a different way, for example solid tissue collected in FFPE and flash formats, or FFPE and fresh tissue sample, or PBMCs and plasma samples. Matched biosamples are a direct indication of the increasing request for customization in biospecimen collection projects that mirror the profound level of sophistication biomedical research has achieved.
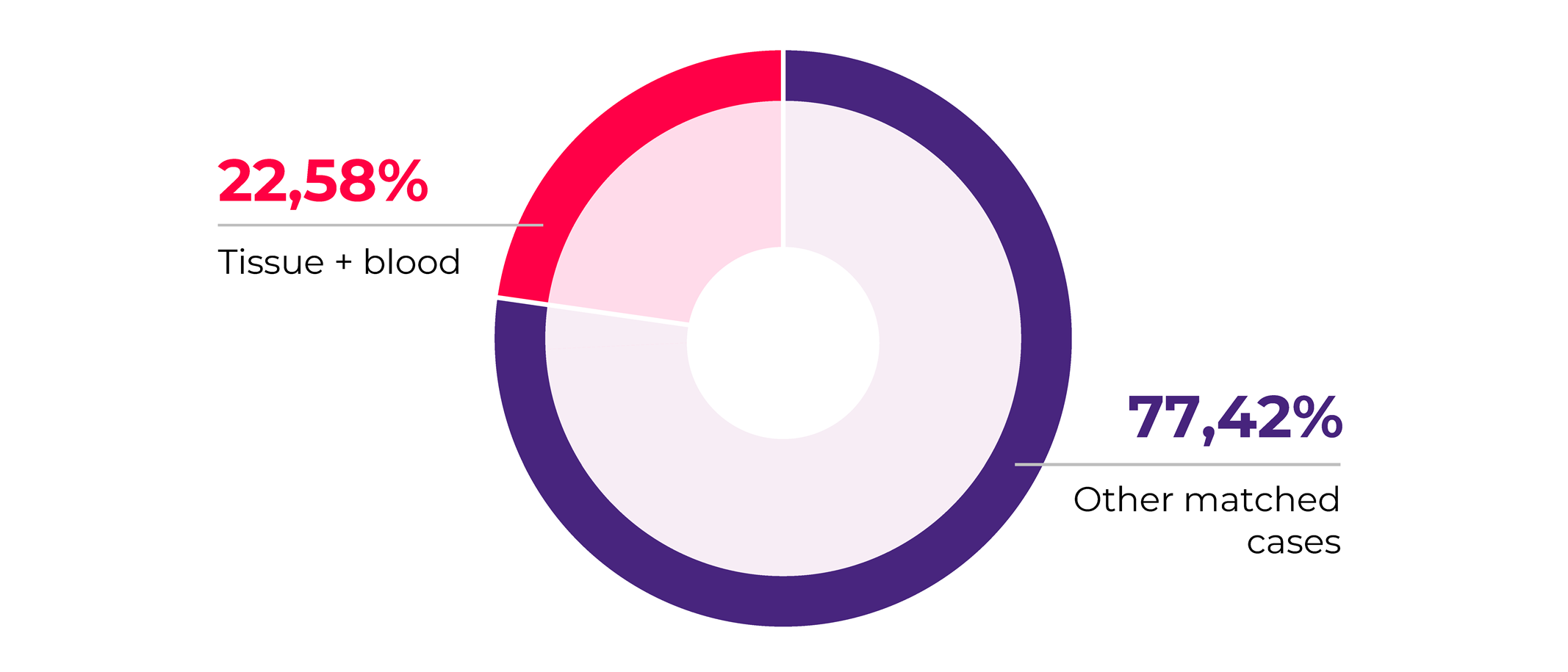
Looking closer into our matched sample collections for 2020 we observed that an important fraction (22.58%) consists of solid tumor tissue matched with patients blood samples. We find this trend to be aligning with the increased interest in liquid biopsy tests development in which scientists are looking for ways to accurately detect and measure tumor signatures in a non-invasive manner from a simple blood sample5. This is further supported by the fact that blood plasma is the most requested biosample in our 2020 collections.
We then moved forward to identify what were the preservation formats researchers were most interested in. Interestingly, we observed that complexity of combinations was thriving. 28.6% of matched blood and tissues collections were interested in collecting FFPEs from tumor tissues together with normal adjacent tissue FFPEs, blood plasma and PMBCs, showcasing the increasing complexity biomarkers studies are having.
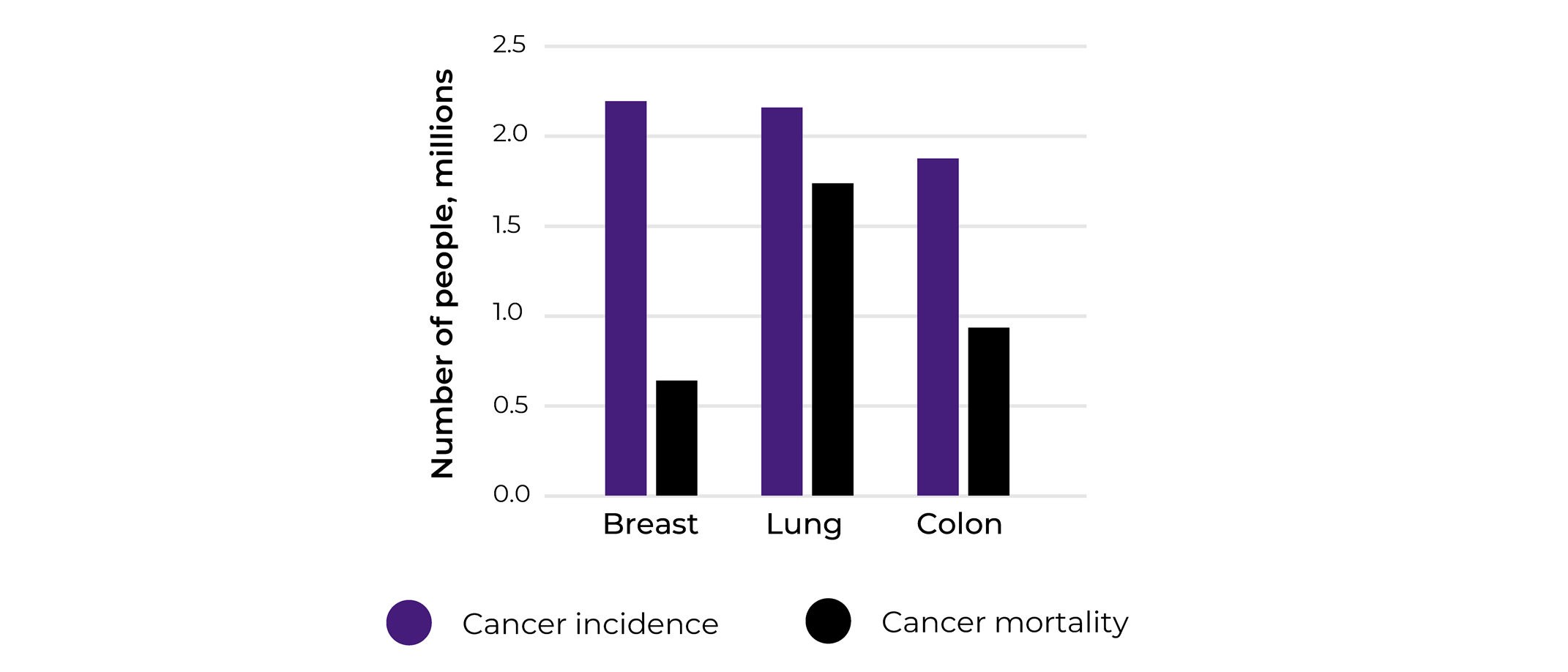
To get a glimpse of what cancer fields were mostly interested in matched biospecimen sets collections and how relevant our collections analysis was, we looked at the cancer types the different projects were focusing on. The main 3 cancer types that we were collecting were breast, lung and colorectal. Indeed, our 2020 statistics align with the WHO cancer incidences statistics for the same year reporting breast cancer to be associated with the highest incidence (2.26 million cases), followed by lung (2.21 million cases), and colorectal cancer(1.93 million cases).
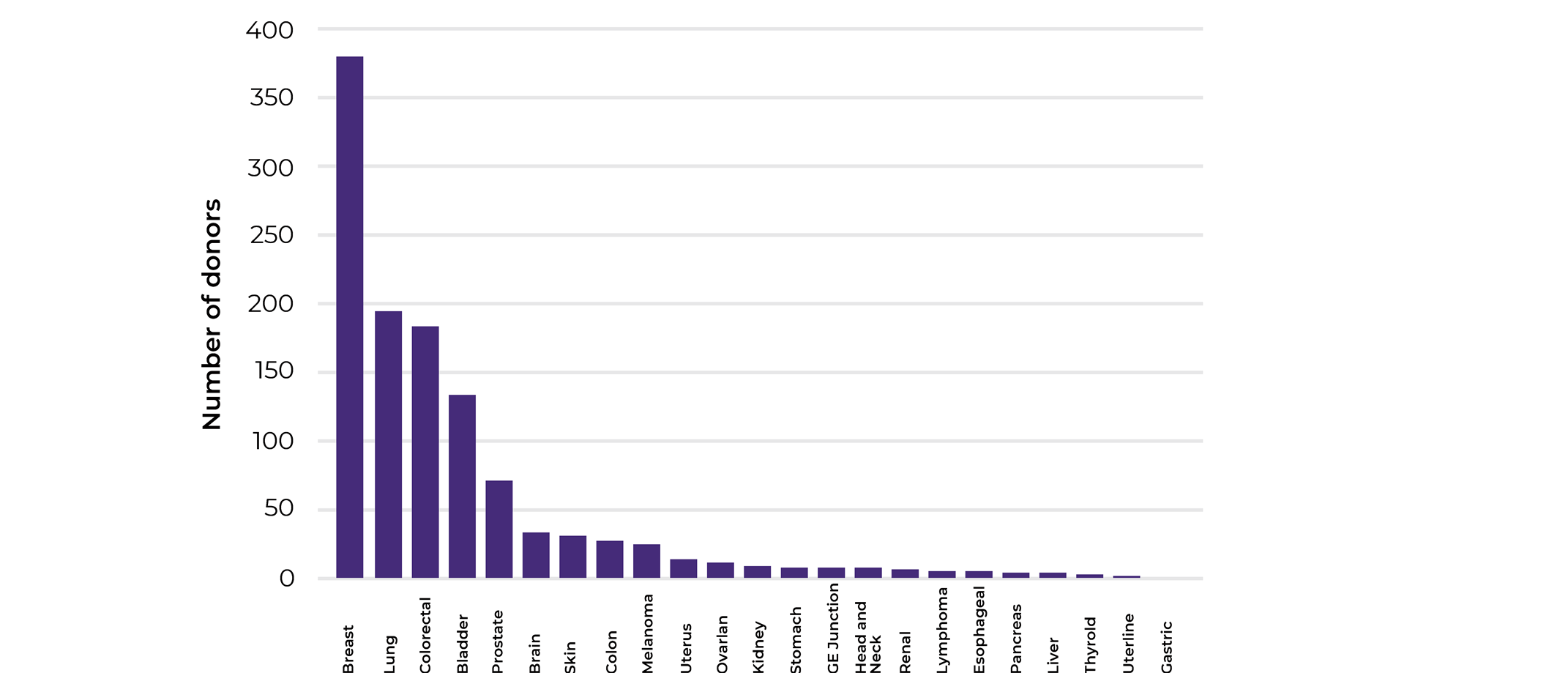
Looking closely into the numbers, while lung cancer comes second in terms of incidence, according to WHO, it is the world leader in mortality with 1.80 million deaths in 2020 in comparison to breast (685 000 deaths in 2020) and colorectal (935 000 deaths in 2020). This is showing that while breast cancer is most prominent cancer type, efficient treatments and early detection are available, this is not the case for of lung cancer patients.
How is our analysis reflecting the urgent unmet oncology needs?
Lung cancer collection projects are strongly presented throughout our 2020 matched sample collections. Seven out of eight projects collecting matched samples are focusing on lung cancer patients. From those three projects are studying cell-free DNA (cfDNA) as a potential biomarker signature and five are focusing on proteomics. Unfortunately, only three out of eight projects request ethnicity data, showing that more efforts towards raising awareness on the subject are needed.
Our observations are in alignment with the Urgent need to improve sensitivity and specificity of lung cancer liquid biopsy tests. Several aspects of this disease contribute to this. Lung cancer is not only the most lethal cancer worldwide, but also it is usually detected at a late stage. The significant diversity of lung cancer mutations among different ethnical groups additionally complicates the assignment of an appropriate targeted therapy<sup>6</sup>. Therefore, great hope is put into the development of specific early detection biomarkers development. Sadly, only two FDA-approved liquid biopsy tests for lung cancer, stressing even further the urgent need to invest more efforts in this direction.
We are convinced that what one of factors that makes us a trusted partner is our capability to comprehensively understand the scientific needs of your projects and suggest solutions on how to tackle biospecimens-associated hurdles and bottlenecks. To do so we always aim to upfront with the research innovations, guidelines, and trends. Our analysis correctly showcases the trends and research needs of the year we’re evaluating. This has been the first similar study we make, and we are performing such analysis now on early bases to compare the trends in our projects and the overall interests and goals in the field of cancer research. Based on our analysis, we are strongly convinced that the degree of customization of biospecimen collections will continue to rise and be increasingly complex, but what we are hoping to note in the years to come is more requirements towards ethnic data.
If your research project needs customized biospecimen collection or you’re developing lung cancer biomarkers, take a look at our matched biosamples collection capacities or get in touch with our team to learn more about how we can help you achieve your research goals.
References:
- Pepe, M. S., Li, C. I. & Feng, Z. Improving the quality of biomarker discovery research: The right samples and enough of them. Cancer Epidemiol. Biomarkers Prev. 24, 944–950 (2015).
- Schully, S. D. et al. Leveraging biospecimen resources for discovery or validation of markers for early cancer detection. J. Natl. Cancer Inst. 107, 1–7 (2015).
- Sirugo, G., Williams, S. M. & Tishkoff, S. A. The Missing Diversity in Human Genetic Studies. Cell 177, 26–31 (2019).
- Financial Times. (2019, January 18). How to stop a lack of diversity undermining clinical trial data. Financial Times. Retrieved February 2, 2022, from https://www.ft.com/content/afd0ac7e-fd3a-11e8-b03f-bc62050f3c4e
- Farber, D. L. Tissues, not blood, are where immune cells act. 4.
- Midha, A. et al. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 9, 2892, (2015).

