Fresh Cancer Tissue Samples – Applications in Biomedical Research
Today, an important research segment of cancer research is interested in fresh cancer tissue samples to extract a particular cell type or molecule or to test the effects of a particular drug or innovative therapy. This biospecimen type offers a mutitude of applications for the most innovative stuidies and projects.
Before researchers delved into the in vitro manipulation of fresh tissues, biospecimens were collected and stored in biorepositories for an indefinite period of time. To make this long-term storage possible, biosamples needed to be preserved. Although these methods are still needed and used, the expansion of innovative biomedical research has reshaped the static nature of biobanking.
Research is not only speeding up in terms of time and efficiency but also in terms of the disease models it uses, which provokes the distancing from the traditional 2D cell culture towards more sophisticated 3D and organoid cultures. The persistent non-responsiveness or resistance detected in some patients are additionally stressing the need for robust in vitro systems and models.
To overcome these issues, scientists are looking deeper into the solid tissue structure to isolate and characterize the unique cellular subsets it contains.
These current research trends affect not only blood samples but also expand largely towards solid tissues. Here we take a look at the applications of using fresh tissues in biomedical research and the popular questions scientists aim to answer using such specimens.
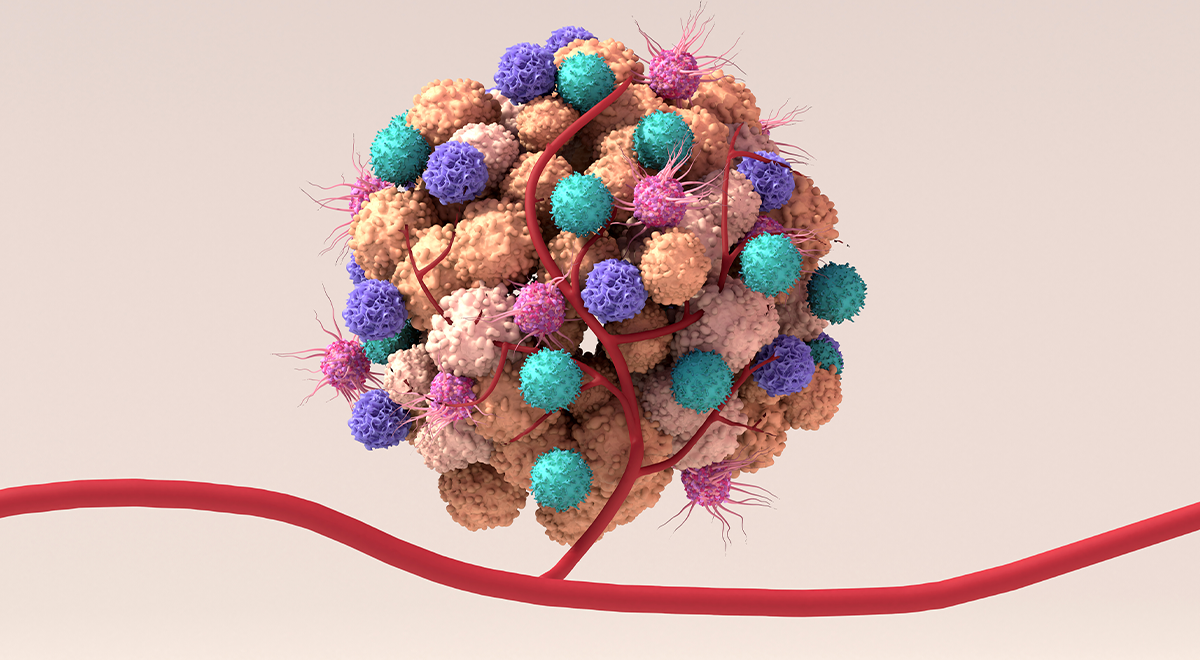
Fresh cancer tissue samples are crucial for understanding the tumor microenvironment
While the overall conception of solid tumors has been of a mutated but uniform mass of cancer cells, today, conversations have primarily shifted towards the diversified cellular nature of tumor formations.
It is well established that solid tumors are composed not only of cancer cells but also non-cancer cells such as endothelial and epithelial blood vessel cells, macrophages, and infiltrated immune cells such as T-cells, the most abundant tumor-infiltrating lymphocytes. The communication of these cell types with the tumor cells has been shown to induce changes in the non-malignant cell types and to play a critical role in cancer development, progression, and response to therapy. Moreover, the heterogeneity of solid tumor tissue is further represented in the various genetic backgrounds of different pools of the solid tumor and the differences between the primary tumor and metastatic formations.
Tackling these genetic variabilities and multiple communications relies strongly on the collection and analysis of fresh tumor tissue. A recent work by Zhang and colleagues published in Nature Medicine is an excellent example of this 1. The team uses tumor tissue matched with blood and normal adjacent tissue from esophageal squamous cell carcinoma (ESCC) to characterize the tumor microenvironment, the interactions between the various cell types and correlate these findings to the patient's responsiveness to immunotherapy.
Matched with blood samples – better insights into the tumor microenvironment and liquid biopsy potential
The same work performed by Zhang et al. nicely uses a combination of biosamples from the same patient to dive deeper into the genetic changes that occurred within the malignant tissues and how they relate to survival and response to therapy 1.
The development of liquid biopsy biomarker tests is an innovative approach. Because of their great promises for non-invasive, fast, and effective disease detection and monitoring, they are met with excitement by the research and clinical communities. Making these promises a reality depends on the correct development of such tests. Knowing which blood or biofluid features are cancer-derived requires the complete characterization of solid tumor tissues. This drives the fact that fresh tumor tissue is often collected alongside blood or biofluid samples in liquid biopsy development studies and speaks volume to our goal of expanding matched biosamples sets for researchers .
Interestingly, it is a critical approach not only for cancer projects. A recently published Comment article by Prof. Donna Farber describes the difference between immune cell profiles from blood and airway wash samples of severe Covid-19 patients. The work discusses the differences at the level of the immune profiles of the two samples, pointing out the need to revise the different pools of immunity across the body to obtain a comprehensive understanding of its immunity system 2. This concept is also valid and transferable to cancer research because of the crucial role of immune cell infiltration within tumors.
Deepen the understanding of the immune system
Indeed, immune cells are an essential part of the tumor microenvironment. The body's immune system becomes alarmed against cancer cells as it does against bacteria or viruses. But as immune cells infiltrate the tumor formation, the cancer cells seek to deactivate them. Therefore, the goal of cancer immunotherapies is to enhance and tune one's immune system against malignant formations.
Over the past decades, the field has achieved impressive success and delivered several life-saving therapies such as immune checkpoint inhibitors, monoclonal antibodies, and CAR-T cell therapies. Unfortunately, some tumors succeed in escaping even from those highly targeted therapies. Due to this, scientists are diving deeper into what is happening within the solid tumor matrix and trying to identify the features that switch off the immune system. Immunophenotyping is a key analytical method for this, and it requires fresh cells as starting material 3,4. This method and the strong focus on the immune cells within the tumor tissue are more reasons for the need for fresh tissue collections.
Moving towards more appropriate in vitro disease models
One of the major limitations translational research faces is the amount of data and findings at the discovery level that fails to be reproduced during the clinical stages. While technological advances are blooming and constantly delivering the next breakthrough, it is becoming more and more evident that one of the main reasons for such failures hides within inadequate disease models. Whether it is a misidentified cell line, an animal model failing to replicate a disease phenotype correctly, or simply a question of the biased nature of in vitro cultures or inadequate pre-analytical handling, alternating biospecimens integrity - the reasons are many, and scientists need to find the right solutions.
As a response to the above-listed limitations, researchers are moving away from the classical 2D cell cultures towards the more native and representative 3D structures. Such models show outstanding results in a number of applications and are opening the doors for personalized drug testing in multiple disease areas from cancer to neurodegeneration 5,6,7.
Developing such innovative and representative disease models is undoubtedly the right way to go for improving translational research. In parallel, growing attention focuses on the importance of good practices when handling and storing biosamples for research purposes.

Collection and processing of fresh tissue
Fresh tissue biospecimens are mainly used to isolate a unique cell type or prepare a smaller 3D structure. Therefore, the tissue needs to be carefully collected within the appropriate culture media and processed within the proper time and temperature limits. Cell isolation requires subsequent digestion or disintegration steps. At this level, the variability of protocols and conditions takes over. The digestion approach, mechanical or enzymatic, will be dictated by the type of tissue. The protocol should also consider the desired cell type as the conditions will differ 4,8.
Although no digestion or fragmentation is necessary when working with blood samples, isolating fresh and functional blood cells comes with more requirements and specifications than the classic buffy coat isolation 9,10.
Closing remarks
At Audubon Bioscience, we follow and analyze the requests and needs of the biomedical field closely. For us, it is vital to understand them in order to be of better service to our clients and, together with them, to contribute to a higher level of healthcare solutions for all patients around the world.
To support the latest translational research directions, we provide a wide range of fresh tissue and biofluids collection services. We understand that having the most appropriate and well-preserved biospecimen is the first step towards efficient and trustworthy research, which will deliver new treatment and diagnostic solutions to patients. For this, we never stop learning in order to ensure the high-quality standards required for fresh tissue samples.
To learn more about our fresh tissue collection capacities, visit our dedicated product page or get in touch with our team.
References:
- Zhang, X. et al. Dissecting esophageal squamous-cell carcinoma ecosystem by single-cell transcriptomic analysis. Nat. Commun. 12, 5291 (2021).
- Farber, D. L. Tissues, not blood, are where immune cells act. 4.
- Corgnac, S. et al. CD103+CD8+ TRM Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep. Med. 1, 100127 (2020).
- Egelston, C. A. et al. Human breast tumor-infiltrating CD8+ T cells retain polyfunctionality despite PD-1 expression. Nat. Commun. 9, 4297 (2018).
- Boucherit, N., Gorvel, L. & Olive, D. 3D Tumor Models and Their Use for the Testing of Immunotherapies. Front. Immunol. 11, 603640 (2020).
- Neal, J. T. et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 175, 1972-1988.e16 (2018).
- Bose, R., Banerjee, S. & Dunbar, G. L. Modeling Neurological Disorders in 3D Organoids Using Human-Derived Pluripotent Stem Cells. Front. Cell Dev. Biol. 9, 640212 (2021).
- Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 10, 3991 (2019).
- Goods, B. A. et al. Blood handling and leukocyte isolation methods impact the global transcriptome of immune cells. BMC Immunol. 19, 30 (2018).
- Gottfried-Blackmore, A. et al. Effects of processing conditions on stability of immune analytes in human blood. Sci. Rep. 10, 17328 (2020).

